The complete interview for actualno.com: https://www.actualno.com/ healthy/news_1616664.html
The European Medicines Agency (EMA) has determined the minimum amounts of nitrosamines that may be part of the medicines for blood products known as ‘sartans’ (angiotensin receptor blockers). This is clear from an official announcement of the EMA.
According to the report, it will now monitor the amount of nitrosamines not only in the active ingredient of the drug, but also in the drug as a whole. In addition, by September 26, 2022, an analysis will be made and there will be a report on the production process and the risk of formation of nitrosamines in the production of sartans. The idea is to reduce as much as possible the risk of nitrosamine formation.
Nitrosamines are chemical compounds that are formed by the reaction of nitrites with amines. Nitrosamines occur under certain conditions – for example, high temperature (certain types of cooking), high acidity (there are such conditions in the stomach). When food nitrites interact with amines in the stomach to form a compound, the risk of stomach cancer increases. In addition, cigarette smoke contains N-nitrosodimethylamine.
Actualno.com contacted the dermatologist Prof. Dr. Georgi Tchernev, one of the best specialists in Bulgaria regarding skin cancer. In a series of materials and interviews Prof. Tchernev commented on the topic of the carcinogenic effect of sartans, and in addition he and his colleagues have a number of publications in specialized foreign scientific sources on the subject. Some time ago he spoke to Actualno.com with the names of manufacturers and their medicines, for which he reported to the Executive Agency for Medicines (BDA)! It turned out that the professor had already published a kind of interview on his personal Facebook profile on the topic.
Prof. Tchernev is adamant that “in practice, the EMA confirms the presence of nitrosamines in blood drugs and at the same time seeks to reduce this availability as a concentration or daily intake ! There is no way to set limits on something that is not established or whose concentration is not This is a kind of self-confession for sleep deprivation or reduced drug control for a period of at least 11 years back in time. ”Why 11 years ago – because 11 years ago Lancet Oncology had a publication on the subject.”
It is difficult to say whether the data and the EMA’s decisions are worrying, as the curtain behind the scenes in the pharmaceutical business is still slightly raised at the moment! But only slightly! However, this gives rise to serious analysis, but it is not yet “fully in the right direction”, although it can be defined as an attempt to reveal part of the truth! But in a forced manner and due to pressure based on scientific publications and data! “, the professor added.
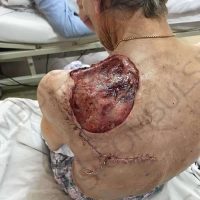
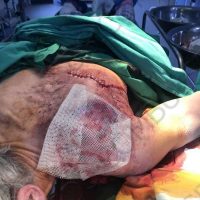
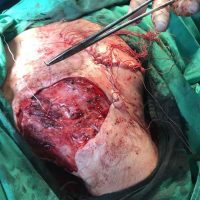
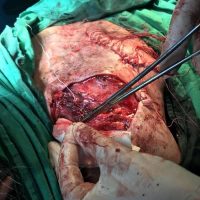
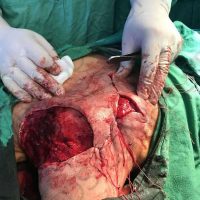
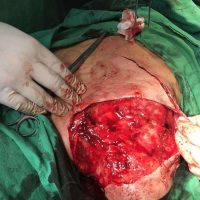
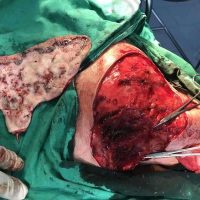
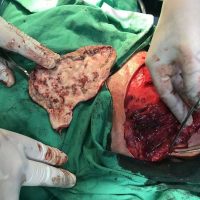
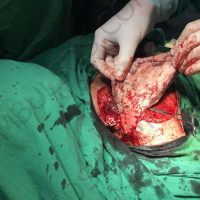
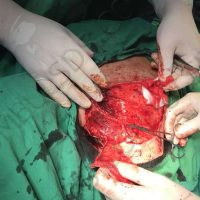
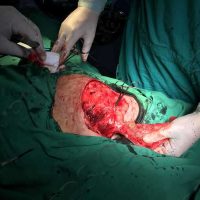
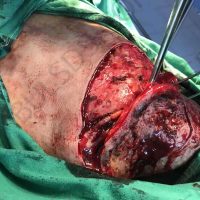
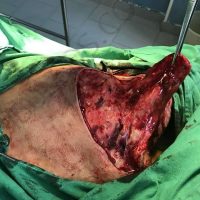
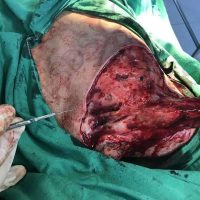
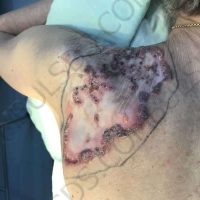
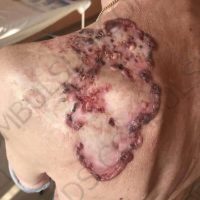
“European drug policy allowed and allows up to now produced in India and China active ingredients to be labelled as made in Europe as long as they are packaged in its territory! This privilege or” bubble, walking to burst “already beginning to burst ! We are looking for arguments to justify the wrong policies and partially correct them, but not eliminate them! This was the basis of our analyzes and findings based on our clinical follow-up – we announced about “20 European companies ” that produce sartans/ angiotensin receptor blockers after the application which develop cancerous forms and in particular melanomas and epithelial skin tumors! Protective behavior that shone and was exposed! Rescue options are currently being sought so that the profits of the big pharmaceutical companies do not fall!”, Prof. Georgi Tchernev is categorical.
The dermatologist also commented that there is currently a debate about what an acceptable dose of nitrosamines means. He reminded that until recently the EMA withdrew all batches that are considered contaminated – such was the case in Bulgaria, from July 6, 2018 ( HERE ), and then, on September 18, batches of valsartan were released after BDA announced that a certain Chinese manufacturer was not involved in the production of their active substance, which is why the initial decision to block the batches was made.
“Now the supposedly contaminated batches were not contaminated enough? Or there is a possibility that the sartans are actually contaminated, but when this contamination with carcinogens / nitrosamines is minimal, they can be accepted ?! Sounds kind of incredible? So with arsenic… if the dose is low, can be taken for years! Poisoning could be slow and chronic, but in practice it remains available!
It is important that the public is informed about the potential risk and has a choice! By initialling minimum permissible doses for nitrosamines, for example, this problem will be somewhat legally justified and addressed, and to some extent companies and control bodies would be protected from subsequent lawsuits! But this protection applies to future, not past periods! “, says Prof. Georgi Tchernev.
“Until last year, the control bodies in the face of the EMA postulated that batches of nitrosamine-containing blood drugs must inevitably be removed from the drug market! Similar to the actions of the FDA (US Food and Drug Administration)! However, we ourselves felt a slight delay in making the right decisions! This had its reason, as can be seen – the creation of dose limits for different nitrosamines in blood drugs! The blame for the pollution was shifted solely to generics from China and India! Our clinical observations over the years have not confirmed these data and we shared in our first international scientific publications the opinion that regardless of whether the respective drugs are generics or originals, sartans generate cancerous forms! That’s when the idea came,”, points out the professor.
Prof. G. Tchernev also considers that it is possible that there were secret inspections of the regulatory bodies regarding nitrosamines and on their basis there is now a permit for the minimum permissible concentration. Once again, he raises the question of who is responsible if a patient actually develops a form of cancer and dies due to taking a drug.
” It is interesting to answer the question: ‘Well, since until now all other manufacturers of sartans(except India and China) did not contain nitrosamines (according to data from EMA / FDA control autorities), now, then the authorization for minimum permissible doses, let the results of the checks for the presence or absence of nitrosamines be announced? I don’t see anything disturbing in that! But it doesn’t happen?!”, explains Prof. G Tchernev.