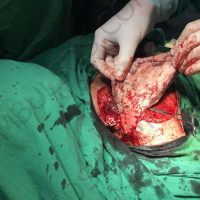
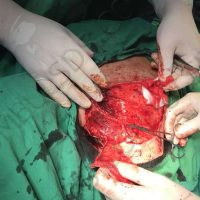
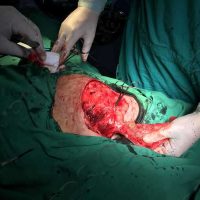
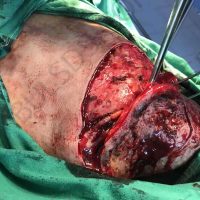
Doctors in Bulgaria indicate that they may have identified the first case of valsartan causing melanoma, a deadly form of skin cancer.
In a case report (PDF) was published late last month in the Macedonian Journal of Medical Sciences, doctors note that the case report comes at a time when numerous valsartan-based hypertension drugs are being recalled due to a risk that they were sold for years with cancer-causing impurities.
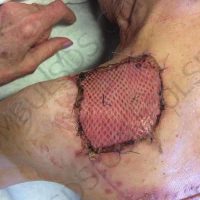
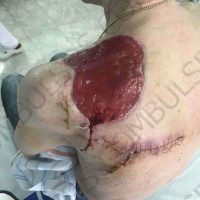
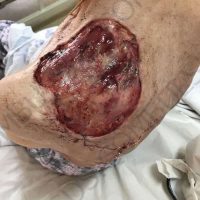
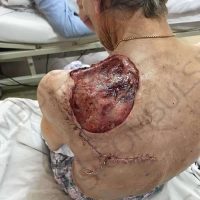
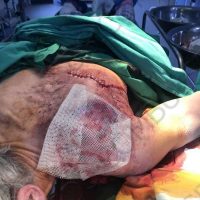
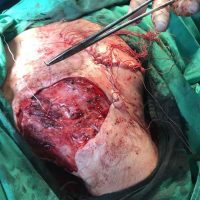
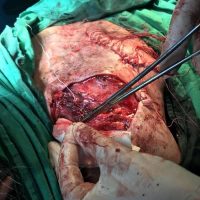
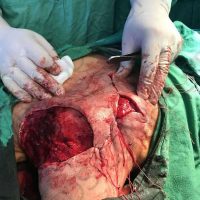
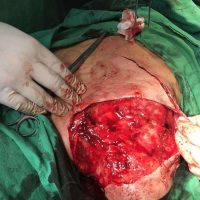
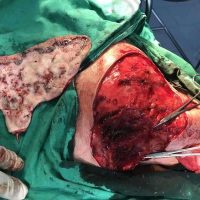
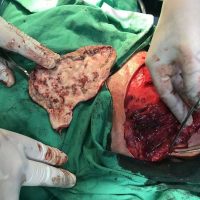
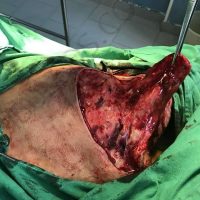
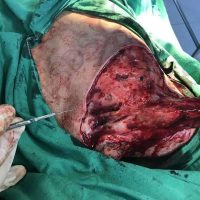
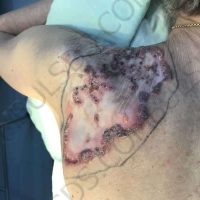
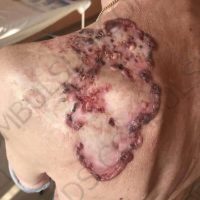
The valsartan melanoma case involved a 70-year-old patient who had taken amlodipine and valsartan since 2008, and valsartan alone since 2015. In 2011, three years after beginning the treatments, the patient developed a pigment lesion on the right arm. After about two and a half years following a doubling of the doseage of valsartan, the legion increased in size, resulting in hospitalization, surgical removal, and the determination that the lesion was melanoma.
“The case presented by us poses several interesting questions: the newly discovered melanocytic lesion occurred 3 years after the first intake of valsartan, as in the last 2-3 years (2015- 2018), the patient observed an increase in its size, which coincides with the introduction of a second product containing valsartan (of the same pharmaceutical company),” the researchers noted. “The inevitable association that occurs is that the progression of melanoma and the likelihood of developing metastases may be dose dependent.”
Researchers note, however, that the particular valsartan-based drugs used by the patient have not been recalled, which could mean that the potential cancer risk is not associated with the potentially cancer-causing impurities, N-nitrosodimethylamine (NDMA) and N-nitrosodiethylamine (NDEA), which have led to dozens of valsartan products being recalled in recent months. However, it could simply mean that the product, manufactured in Germany, just has not been recalled yet, as valsartan recalls have continued into the new year.
“This means that the carcinogenic effect may not only be related to the presence or contamination with NDMA but may come directly from the generic substance of valsartan as well as from the presence of a potential another carcinogen,” the researchers warned.
Valsartan Recalls
The first concerns about the risk that valsartan pills may contain impurities that increase the risk of cancer surfaced in July, when European regulators announced that batches of the active ingredient supplied by Zhejiang Huahai Pharmaceuticals tested positive for NDMA. The FDA followed with its valsartan recall announcement on July 13, indicating that the agency had launched an investigation to determine the scope of the contamination and the potential risk to consumers.
In late September, the FDA stopped all imports of drug ingredients and medicines made by Zhejiang Huahai Pharmaceuticals, after an inspection report outlined a number of serious manufacturing problems at the company’s facility in late August.
The recalls have led to a valsartan shortage and a spike in prices, with the cost of 160 milligram and 80 milligram tablets of generic valsartan more than doubling in September 2018.
As consumers nationwide continue to face concerns about the safety of pills they have taken in recent years, a number of valsartan recall class action lawsuits have been filed nationwide, seeking damages for the cost of the recalled drugs and medical monitoring. For those diagnosed with liver cancer, kidney cancer, pancreatic cancer, stomach cancer and other cancers, individual cases are being reviewed by valsartan lawyers.
The FDA has assigned a group of pharmacists and nurses to answer consumer’s questions about the recalls. Since the first recalls, the agency indicates it has received more than 6,000 inquiries from patients, doctors, nurses, pharmacists and academics. Inquiries can be made by calling 855-543-3784 or by sending an email to druginfo@fda.hhs.gov.
Source: Irvin Jackson, aboutlawsuits.com